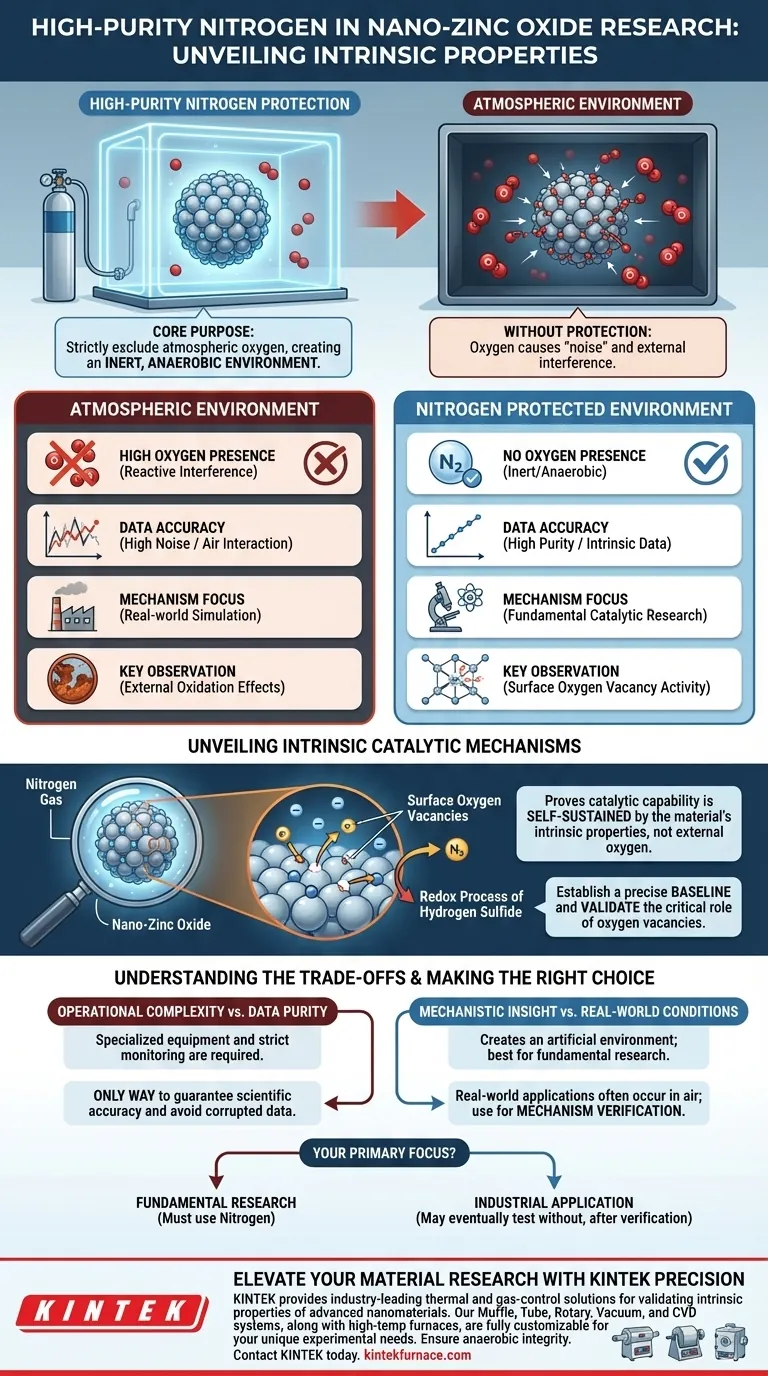
The primary function of a high-purity nitrogen protection device is to strictly exclude atmospheric oxygen during the surface modification and performance testing of nano-zinc oxide. By creating an inert, anaerobic environment, this device ensures that any observed chemical activity is the result of the material's intrinsic properties rather than external air interference.
By eliminating external oxygen, researchers can establish a precise baseline. This reveals that surface oxygen vacancies alone can drive the redox process of hydrogen sulfide, proving the material's catalytic capability without reliance on atmospheric support.

The Critical Role of Environmental Isolation
Eliminating Atmospheric Interference
The atmosphere contains oxygen which is highly reactive. In standard testing environments, this external oxygen can interact with the nano-zinc oxide surface.
This interaction introduces "noise" to the data. Using high-purity nitrogen effectively displaces this oxygen, removing it as a variable in the experiment.
Establishing an Anaerobic Baseline
To truly understand a catalyst, you must test it in a vacuum or inert state. The nitrogen protection device creates a verified anaerobic environment.
This allows researchers to simulate conditions where no external oxidizers are present. It ensures that the chemical reactions observed are self-sustained by the material itself.
Unveiling Intrinsic Catalytic Mechanisms
Observing True Catalytic Behavior
The core purpose of this setup is to observe the true catalytic behavior of the nano-zinc oxide. specifically focusing on its surface structure.
Without nitrogen protection, it is impossible to distinguish between reactions caused by the material and those facilitated by ambient air.
Validating the Role of Oxygen Vacancies
Nano-zinc oxide possesses specific structural defects known as surface oxygen vacancies. These vacancies are critical to the material's performance.
Experiments under nitrogen protection demonstrate that these vacancies facilitate the redox process of hydrogen sulfide. They do this by capturing electrons, a process that occurs even in the total absence of external oxygen sources.
Understanding the Trade-offs
Operational Complexity vs. Data Purity
Using a nitrogen protection device adds a layer of complexity to the experimental setup. It requires specialized equipment and strict monitoring of gas purity levels.
However, this added effort is the only way to guarantee scientific accuracy regarding the material's intrinsic mechanisms. Skipping this step risks gathering corrupted data driven by atmospheric contamination.
Mechanistic Insight vs. Real-world Conditions
While this device is essential for understanding how the material works, it creates an artificial environment.
Real-world applications of desulfurization often occur in the presence of air. Therefore, this setup is best used for fundamental research and mechanism verification rather than simulating final industrial operating conditions.
Making the Right Choice for Your Experiment
To determine if this setup is necessary for your work, consider your specific analytical goals:
- If your primary focus is fundamental research: You must use nitrogen protection to prove that oxygen vacancies are the active sites driving the reaction.
- If your primary focus is industrial application: You may eventually need to test without protection to see how the material performs in ambient air, but only after the intrinsic mechanism is verified.
The use of high-purity nitrogen is not just a procedural step; it is the definitive method for confirming that your nano-zinc oxide possesses self-sufficient catalytic power.
Summary Table:
| Feature | Atmospheric Environment | Nitrogen Protected Environment |
|---|---|---|
| Oxygen Presence | High (Reactive Interference) | None (Inert/Anaerobic) |
| Data Accuracy | High Noise (Air Interaction) | High Purity (Intrinsic Data) |
| Mechanism Focus | Real-world Simulation | Fundamental Catalytic Research |
| Key Observation | External Oxidation Effects | Surface Oxygen Vacancy Activity |
| Purpose | Industrial Application Testing | Validation of Self-Sustained Redox |
Elevate Your Material Research with KINTEK Precision
Precise control over your experimental atmosphere is critical for validating the intrinsic properties of advanced nanomaterials. KINTEK provides industry-leading thermal and gas-control solutions tailored for high-stakes laboratory research.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, along with other lab high-temp furnaces—all fully customizable to meet your unique experimental needs. Whether you are studying oxygen vacancies in nano-zinc oxide or developing new catalysts, our equipment ensures the anaerobic integrity your data demands.
Ready to achieve superior scientific accuracy? Contact KINTEK today to discuss your custom furnace requirements.
ビジュアルガイド
参考文献
- Chunhong Shao, Xiu‐Li Yang. Study on the Surface Structure of Nano-ZnO Desulfurizers and Their Performance and Mechanism in H2S Removal at Room Temperature. DOI: 10.3390/catal15060547
この記事は、以下の技術情報にも基づいています Kintek Furnace ナレッジベース .
関連製品
- 真空熱処理焼結炉 モリブデンワイヤー真空焼結炉
- 2200 ℃ 黒鉛真空熱処理炉
- 真空ステーションCVD装置付きスプリットチャンバーCVD管状炉
- セラミックファイバーライナー付き真空熱処理炉
- 真空シール連続作業回転式管状炉 回転式管状炉
よくある質問
- SHSによる炭化ホウ素チタンの合成において、希釈剤としてのNaClの添加はどのような役割を果たしますか?マスターナノパウダー合成
- 乾燥ヨーグルトの化学組成を決定する上で、制御式熱風循環オーブンの役割は何ですか?
- Li6MnO4前駆体の合成にガス流量制御を備えた高温炉が必要なのはなぜですか?精密合成を実現
- h-BNターゲットはどのようにしてメモリスタのスイッチング比を向上させるのか?高純度前駆体で論理ウィンドウを最大化する
- リン化硫黄薄膜にPVDを使用する意義は何ですか?オプトエレクトロニクス研究を産業規模に拡大する
- グラフェンナノシートの準備において、熱分解炉はどのような役割を果たしますか?高価値プラスチック変換をマスターする
- ゆっくりした熱分解プロセスにおける固定床反応炉の役割は何ですか?高品質バイオ炭製造のエンジニアリング
- ボックスヒーターはどのように機能するのか? 部屋全体を効率的に暖めるためのガイド



















